
In September 2018, we obtained manufacturing/marketing approval for this drug with the effect of “reduction of clinical signs associated with acute pancreatitis in dogs”.
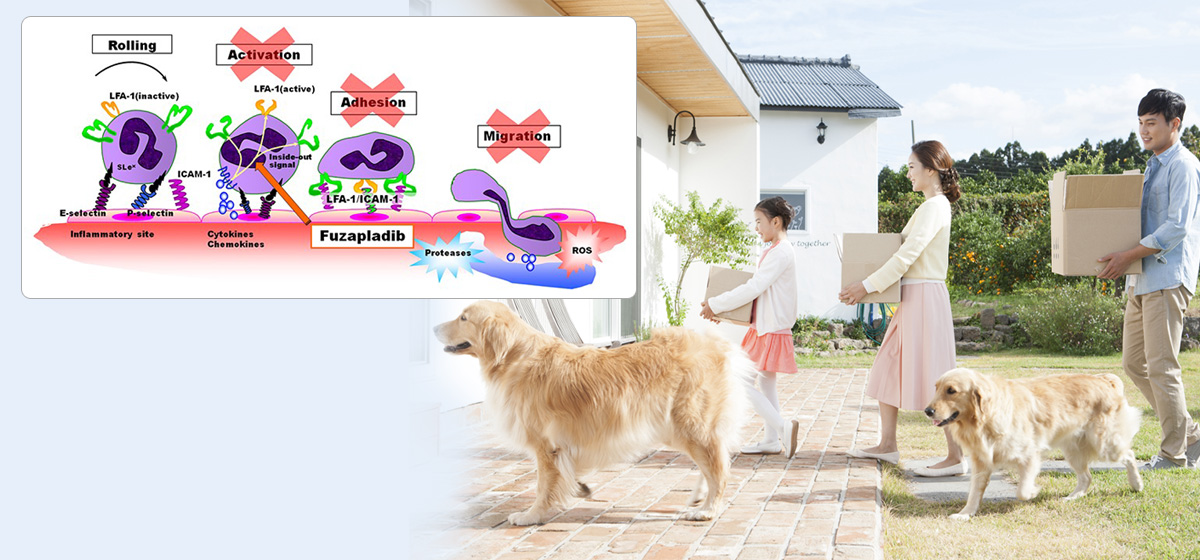
Fuzapladib blocks activation of adhesion molecules (integrin) expressed on the inflammatory cell surface to prevent inflammatory cells from adhering to vascular endothelial cells and infiltrating tissue and to control exacerbation of pancreatitis.
This mode of action is different from that of conventional anti-inflammatory drugs including steroids and non-steroid anti-inflammatory drugs (NSAIDs).
No veterinary drugs with a similar action mechanism is available anywhere in the world, including Japan.
 |
|
| IUPAC name | N-[2-(ethylsulfonylamino)-5-(trifluoromethyl)-3-pyridinyl] cyclohexanecarboxamide mono sodium salt monohydrate |
| Molecular weight | 419.40 |
| Molecular formula | C15H19F3N3NaO3S・H2O |
| CAS RN | 351999-87-6 |
“Fuzapladib sodium hydrate” is an active pharmaceutical ingredient contained in “BRENDA™Z”.
ISK manufactures “fuzapladib sodium hydrate” and supplies it as an active pharmaceutical ingredient for animal use to Nippon Zenyaku Kogyo Co., Ltd.
ISK also obtained the manufacturing/marketing approval for “fuzapladib sodium hydrate” as the trade name, “BRENDA™”.